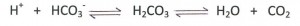Exploring Medicine – A Foreword
I remember clearly the first meeting I had with Steve Jameson in summer 2005. I had just returned from a regional meeting of the National Association of Advisors in the Health Professions (NAAHP) where a number of sessions had focused on the importance of having health professions advisors build partnerships with health care practitioners in the community. So, when approached by Steve I was more than glad to meet to discuss what I thought was merely an opportunity to enhance our students’ access to shadowing opportunities.
Although that original meeting has led to much greater student access to clinical experiences, then I had no idea that it was the launching pad for an innovative educational experiment in the health sciences that has deeply contributed to the academic, professional, and personal development of hundreds of students – Exploring Medicine. In our first conversations in 2005, Steve proposed to teach a course at the College of Saint Benedict/Saint John’s University (CSB/SJU) that would show students how to critically think like a doctor and how to apply the material they were learning in basic science courses to the process of clinical diagnosis.
We eagerly supported Steve’s idea and in spring 2006 Exploring Medicine was taught for the first time. Since then it has been taught every spring at CSB/SJU and more recently in the fall semester at the University of St. Thomas. Exploring Medicine is truly a gem since it allows students to critically engage in a focused and structured learning experience from a clinical standpoint. Through Steve’s interactive lectures students develop analytical skills that allow them to experience the thinking process of a clinician making a diagnosis; through the panels of healthcare professionals and guest speakers students gain an appreciation for the diversity of the healthcare field and the necessity for a team-approach in care; and last but not least, through the structured shadowing experiences provided by the class students can see how principles covered in the course are directly applied in the process of diagnosis and patient care. Indeed so many students continue to pursue the relationships they have established with clinicians in Exploring Medicine that at CSB/SJU, with Steve Jameson’s help, we established a year-long internship program at the St. Cloud Hospital Emergency Department entitled the Student Health Assistant Program. Engaging community physicians to teach at local universities pays dividends, and Exploring Medicine is the ideal platform to establish those relationships.
The uniqueness of Exploring Medicine is that it is not static – Steve Jameson is continuously editing and tailoring his presentations, creating new experiences for students, and providing novel leaning tools and settings. Since 2006 Exploring Medicine also came to include this book – it is used for the class, but many Exploring Medicine alums will also vouch for its continued value as a refresher and review tool in their further studies. More recently Steve has developed online resources that allow Exploring Medicine to be delivered in its unique and creative fashion to health professions students in any college campus throughout the country; tools that were recognized by the AAMC with its 2013 iCollaborative award in biology.
In short, Exploring Medicine is much more than a course, a book, or a set of online tools. Exploring Medicine is a unique experience that allows health professions students to directly bridge their academic background to a structured clinical setting, and to begin to experience the intellectual world as seen through the eyes of a physician. In the process of helping Steve implement his vision at CSB/SJU, I have seen hundreds of students who are now working as physicians, physician assistants, physical therapists, among others engage in their first meaningful clinical discovery in that setting. Exploring Medicine is indeed the bridge from the world of the humanities, social and natural sciences of our college campuses to the experiential setting of clinical medicine and practice.
Manuel Campos, Ph. D.
Professor of Biology, Preprofessional Health Advisor College of Saint Benedict|Saint John’s University
Preface
Choosing a career is one of the most important decisions you will make in your life. While a career in health care, and in medicine in particular, can be incredibly exciting and rewarding, the journey to that end can be an enormous physical, emotional, and financial drain. The decision to go into this field must be an informed one.
Many are enamored with the medical field and with “being a doctor” long before they know much at all about the practice of medicine. Some are told, “You’re smart; you should be a doctor.” Others simply like what they see on TV. The Exploring Medicine series of modules will primarily focus on what it is like to be a physician, but the information is relevant to all that are seeking a career as a health care professional. To make an informed decision regarding a career in medicine, you should first explore the medical field by seeing what physicians do and learn to think like a physician.
This module, and others in this series, will allow you to do just that. Starting with the very first topic, you will plunge into the world of clinical practice. There you will find patients with a variety of medical problems (based on actual cases), many with life-threatening and life changing emergencies that you will need to work through and solve in order to save your patient’s life and make them well. By the time you have finished this module you will have learned to think and problem-solve like a doctor, and you will understand the process of diagnosing and treating disease.
The medical model is all about diagnosis and treatment of disease, and in order to understand that you have to understand pathophysiology. Pathophysiology is the study of disease. The pathophysiologic process of a disease is the series of events that take place and conditions that develop that result in a particular disease. In order to treat disease, the physician must understand something about the pathophysiologic process that led to the development of that disease. By going through the systems based modules you will gain insight into the thought process physicians use to diagnose and treat disease, and how they help patients to become well. Each system based module begins with a general overview followed by a discussion of the pathophysiology of a particular disorder: e.g. the Respiratory module reviews anatomy and physiology of the Respiratory System, then goes into a discussion regarding the cause of asthma and its treatment. Other modules, like Social Determinants of Health, focus on wellness as it relates to society, social factors, and public policy. Exploring Medicine modules were made for the students that have taken biology, chemistry, sociology, psychology, and other science courses, and wondered, “Why do I have to learn this stuff?” These modules apply the student’s science knowledge to the real world by making correlates to clinical medicine.
Books have been written on what will be small elements of the Exploring Medicine modules, so we obviously cannot comprehensively cover every topic. The authors have distilled a massive volume of material down to several manageable lessons. Despite this editing, the amount of subject matter may still seem intimidating at first, but that is not the intent of the authors. Do not get stressed or anxious. The focus of these modules will be on general principles and concepts. In order to transition from simple anatomy and physiology to treatment of disease, for example, we must streamline our approach in order for the reader to get some appreciation of what it is like to be a physician. The following module will take you, the medical enthusiast, through a very narrow slice of medical training: from medical school, to residency training, to clinical practice. Armed with your newly found knowledge, you will be able to make clinical decisions based on true-to- life patient scenarios and help your patients become well or stay well. If you find this process compelling, as the authors do, then you may seriously want to pursue the exciting field of medicine.
Introduction to the Chapters – Life and Death, Homeostasis and Equilibrium
Death is not the enemy but occasionally needs help with timing. – Peter Safar, M.D. The “Father” of CPR
What is life? That may seem like a metaphysical question, but it’s actually not. It is a very real question for those that provide medical care, but the answer can be surprisingly complicated. As a physician, physician assistant, nurse, or nurse practitioner, you will likely have many occasions where you will be dealing with patients as they look to cross that line from life to death. In the following chapters we will discuss a variety of pathophysiologic processes that will alter the normal function of the human body.
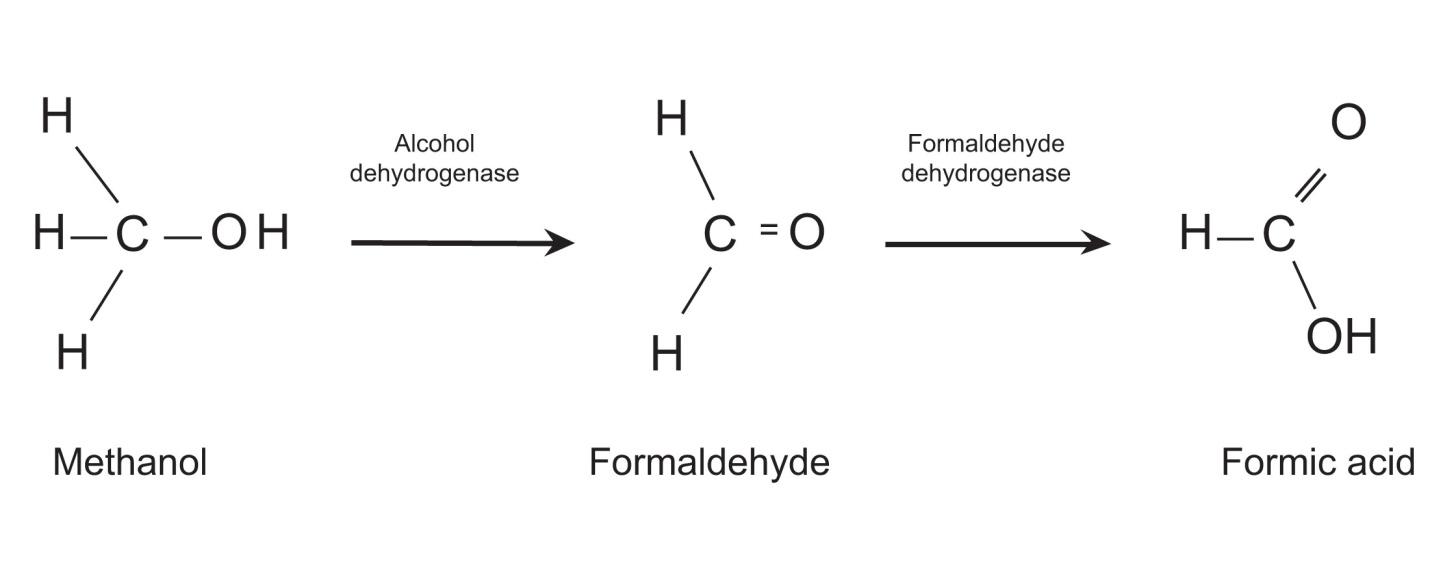
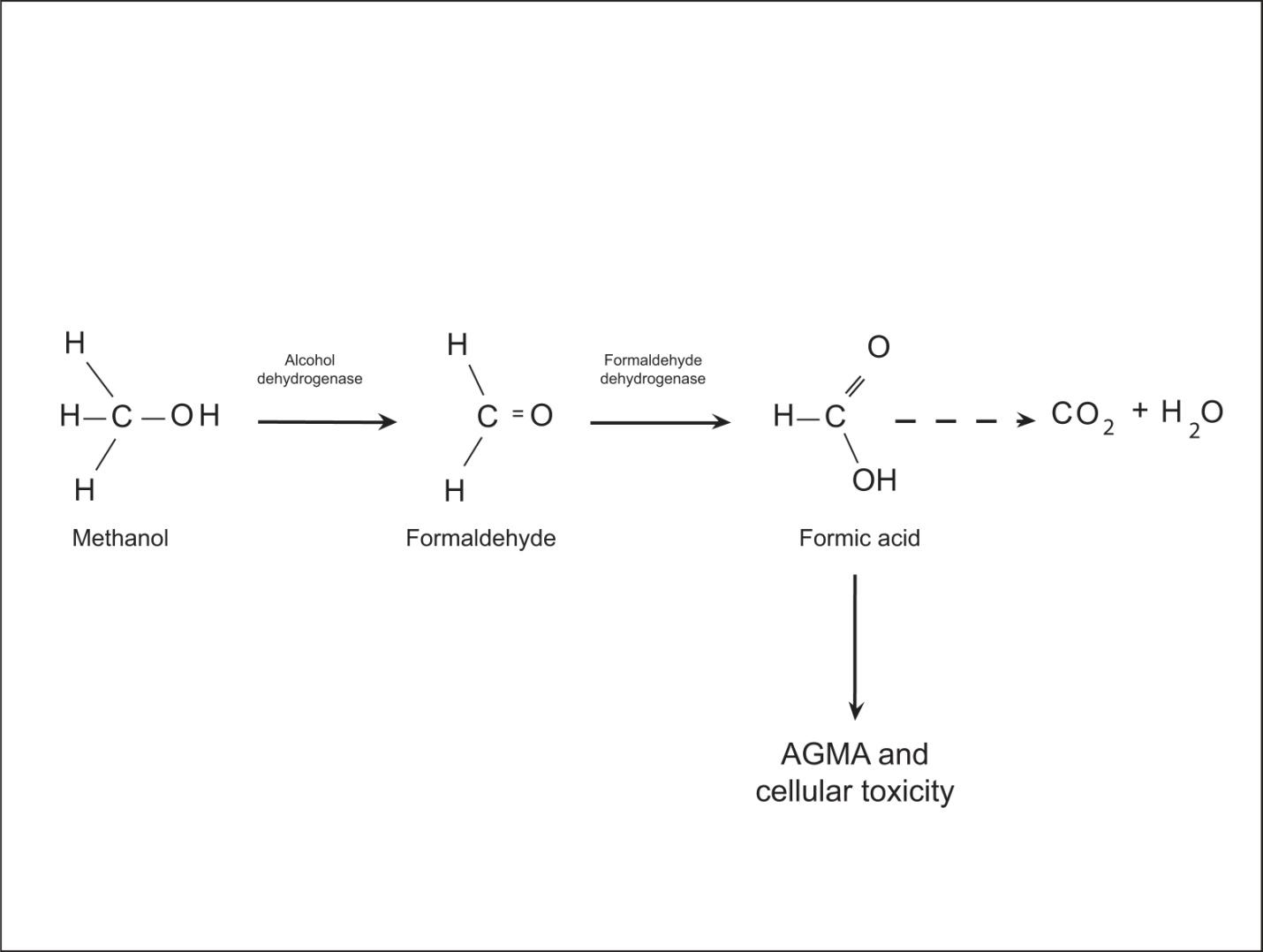
Before we delve into specific disease processes, though, let’s take a look at a more fundamental principle of human physiology and how a disruption of normal processes can lead to chaos, i.e. entropy, on a microscopic (actually molecular) level and to death on a macroscopic level.
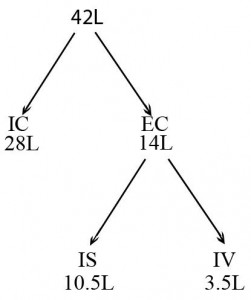
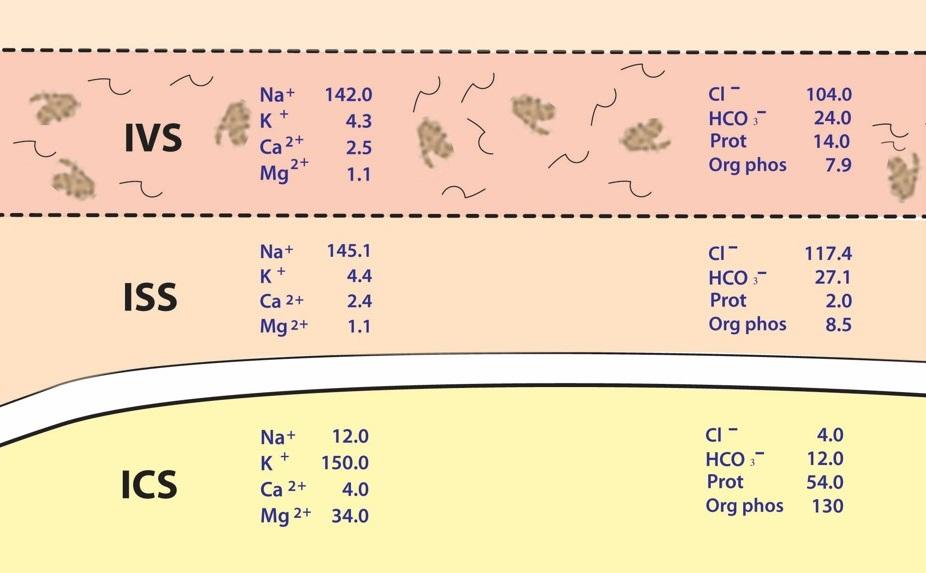
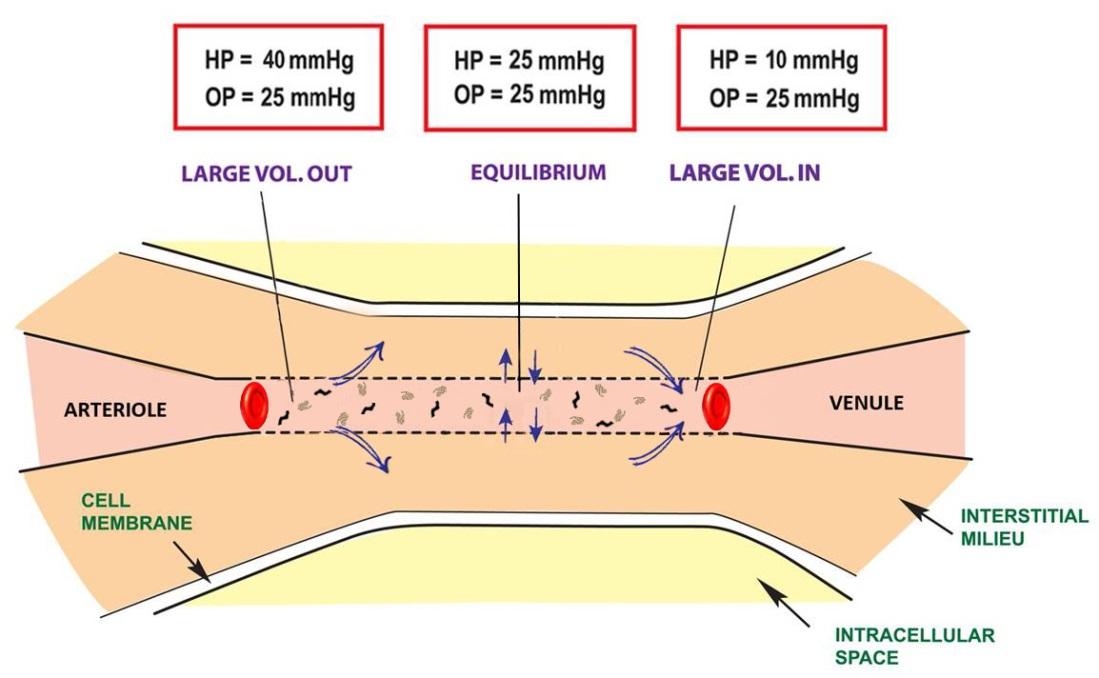
The human body is a complex network of systems that operate in unity to keep us alive and active. In order to maintain normal function the body seeks homeostasis and defies equilibrium. Cellular reactions take place under very specific conditions: a narrow range of pH, the correct concentrations of particular ions, proper amount of substrates, etc. Homeostasis is the set of conditions that the body establishes and maintains in order to function properly. It takes energy to maintain this homeostasis. This energy has to be consumed in the form of chemical bonds contained in the food we eat, converted to a usable form, transported throughout the body to all of its cells, and metabolized into a form of energy the cells can use: ATP. The wastes of these reactions then have to be removed. It is a complex network of integrated systems that accomplishes all of these tasks.
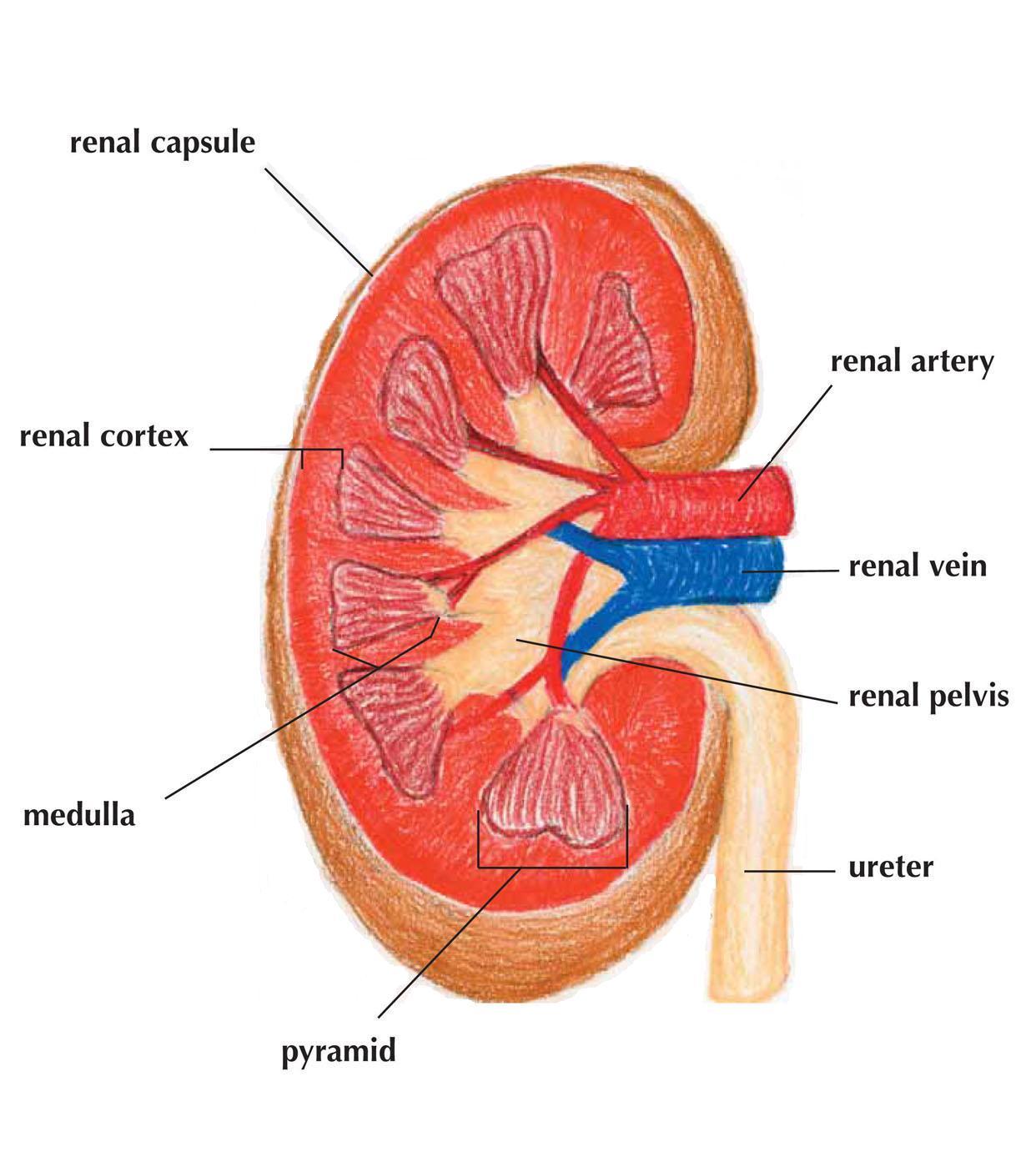
In Exploring Medicine, we will look at four of the body’s integrally related physiologic systems (Cardiovascular, Respiratory, Renal and Integumentary) as examples of how the body maintains homeostasis. We also explore what can happen when these systems fail, and what we, as medical care providers, do to restore normal function and reverse the process of dying. All of the chapters have questions to answer along the way and challenging scenarios to solve at the end of each chapter. Answers to these questions and further elaboration of concepts are in the appendices near the end of the book. In the chapter on Evidence Based Medicine, we will discover how the medical literature helps to guide a physician’s clinical practice. As you strive to learn more about the field of medicine, remember the words of Hippocrates (the final line of the Hippocratic Oath), “May I always act so as to preserve the finest traditions of my calling and may I long experience the joy of healing those who seek my help.”